Advertisement
Definition and Etiology
Schizophrenia is a chronic and disabling neuropsychiatric illness possibly best characterized as a syndrome rather than as a single disease entity. The abnormal, often bizarre behavior that typifies schizophrenia is a product of disturbances in cognition, perception, and volition. Clinical manifestations are believed to result from incompletely understood dysregulation of frontotemporal and limbic neurocircuitry. The National Alliance on Mental Illness (NAMI), a patient- and family-oriented self-help group, has designated schizophrenia a brain disorder, emphasizing that schizophrenia is not simply a product of dysfunctional parenting or other psychosocial stressors. Studies have consistently shown, however, that both genetic and nongenetic factors play a role in the origin of schizophrenia.
Prevalence and Risk Factors
The point prevalence of schizophrenia is 1% to 1.5%, a finding that has been fairly constant across time, cultures, races, and continents. It is equally prevalent in men and women. In the United States, about 2.5% of total annual health care expenditures are for schizophrenia. Globally, schizophrenia is a leading cause of disease burden and disability. The lifetime risk of suicide is nearly 7% compared with 14% to 15% for mood disorders such as major depression and bipolar disorder.¹
The familial nature of the illness has long been recognized. Mounting evidence supports a strong genetic contribution, but genetic factors alone do not fully account for the variance in cause. As with other common illnesses such as hypertension, the risk of developing schizophrenia is a product of multiple genes interacting not only with one another but also with environmental factors. It is also possible that specific risk factors predict occurrence of specific schizophrenia subtypes.
Genetic Risk Factors
Accumulating evidence shows that genetic and neurodevelopmental factors are associated with greater susceptibility to schizophrenia. According to twin and adoption studies, up to 50% of identical (monozygotic) twins share a diagnosis of schizophrenia, compared with about 12% of nonidentical (dizygotic) twins. The strength of genetic factors varies across families, but approximately 10% of a patient’s first-degree relatives (parents, siblings, and children) are also schizophrenic, as are 50% of the children of two schizophrenic parents. Reports indicate suggestive linkage on chromosomes 1, 2, 3, 5, and 11 and on the X chromosome.
Season of Birth
The birthrate of patients with schizophrenia is 5% to 8% higher worldwide than the birthrate of the general population in the winter and spring months. No proven explanation exists for this phenomenon. A greater likelihood of viral exposure during winter months has been proposed.
Early Developmental Insults
A comparatively high rate of peripartum infant hypoxia has been associated with structural brain abnormalities (e.g., increased ventricular and decreased hippocampal volumes) in schizophrenic patients and their nonschizophrenic siblings.
Other Factors
Population density, industrialization, emigration, and low socioeconomic status at birth have been proposed as possible influences on the development of schizophrenia.
Pathophysiology and Natural History
Gross inspection of the schizophrenic brain reveals no abnormalities. Modern neuroimaging techniques, however, including computed tomography (CT), magnetic resonance imaging (MRI), functional MRI, and positron emission tomography, demonstrate evidence of nonspecific structural and metabolic abnormalities in the frontotemporal cortices, especially in the prefrontal areas and periventricular limbic structures of the schizophrenic brain. There have been some correlates of gray matter changes in the left dorsolateral prefrontal cortex in patients with predominantly negative symptoms. Detailed postmortem analysis of protein profiles and metabolic patterns in the brains of schizophrenic patients point to mitochondrial dysfunction as a distinctive feature.2
Neural transmission has long been an object of investigation in schizophrenia. The first agents to demonstrate promise in the pharmacologic control of schizophrenia were recognized to have dopamine-blocking properties. Several neurotransmitter systems have been implicated, but the primary focus has been on dopamine and the brain structures that are high in its content (substantia nigra, ventral tegmentum, mesolimbic structures, and the tuberoinfundibular system). Five dopamine receptor subtypes (D1 through D5) have been identified. Blockade of the D2 receptor appears to have the greatest relevance to the antipsychotic efficacy as well as adverse effects of neuroleptic drugs. The site of D2-receptor blockade is also relevant to its benefits and adverse effects. Extrapyramidal symptoms can be attributed to D2-receptor blockade in the substantia nigra and ventral tegmentum, positive symptom suppression to D2 blockade in mesolimbic structures, and hyperprolactinemia to D2 blockade in the tuberoinfundibular structures (dopamine is a prolactin-inhibiting factor). The relationship to schizophrenia of serotonin, glutamate, gamma-aminobutyrate, neurotensin, and their relevant receptors is also under investigation.
Advertisement
Signs and Symptoms
Although not described as a separate entity in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),2 acute psychosis refers to a symptom complex that includes disturbance of thought processes and behavior. The presence of psychotic symptoms usually indicates an underlying organic or psychiatric condition. Disruption of thought processes, hallucinations, delusions, agitation, and rapid deterioration in behavior are some of the common manifestations of acute psychosis. Acute psychosis can be a feature of schizophrenia, but the diagnosis of schizophrenia requires the fulfillment of a variety of other diagnostic criteria.
The median age at onset for the first psychotic episode of schizophrenia is the early to mid-20s for men and the late 20s for women. A prodromal phase that lasts months to years can precede the first psychotic episode. Acute psychosis, the hallmark of the acute phase, follows the prodrome insidiously or occurs abruptly and sometimes explosively. The natural history without treatment (and sometimes with) is for symptoms to wax and wane, punctuated by recurrent episodes of acute psychosis. The pattern of symptoms can change over time, with progressive deterioration of function and cognition in some instances and progressive improvement of psychotic symptoms and function in others. Full recovery is uncommon, especially if the illness has been present for some years. Comorbid substance abuse is common, prolongs the illness, and contributes to treatment resistance.
The prodromal phase of schizophrenia is characterized by social avoidance, emotional flattening, eccentricity or magical thinking, idiosyncratic speech, and peculiarities of attitude and behavior that fail to meet criteria for a specific psychiatric illness. Prodromal symptoms that suggest social anxiety, panic, obsessive-compulsive or major depressive disorder, and antisocial behavior or substance misuse often lead to early misdiagnosis and unsuccessful treatment efforts.
Factor analysis has identified three main psychotic symptom dimensions in schizophrenia: positive, negative, and cognitive. The acute phase of the illness features a predominance of positive psychotic symptoms, whereas the chronic phase is typified by negative and cognitive symptoms. Unlike other types of psychosis, the positive symptoms of schizophrenia are complex and bizarre (i.e., having to do with unreal or unearthly events). Negative symptoms are believed to reflect neuroimaging evidence of reduced metabolic activity in the dorsolateral prefrontal cortex. Positive symptoms might represent abnormal temporal lobe activity. Characteristic features of positive, negative, and cognitive symptoms are outlined in Box 1.
Box 1. Schizophrenia Symptoms and Symptom Dimensions
| Positive* |
| Hallucinations (typically auditory but also visual) |
| Delusions (paranoid delusions, nihilistic delusions, delusions of control) |
| Unusual behavior (stereotypies, mannerisms) |
| Negative† |
| Reduced emotional (affective‡) range |
| Diminished speech production (poverty of speech) |
| Loss of interest (anhedonia‡) |
| Loss of drive, initiative (apathy, abulia‡) |
| Indecisiveness (ambivalence‡) |
| Cognitive† |
| Poor attention |
| Working memory impairment |
| Formal thought disorder (tangential thinking, loose associations)§ |
| Concrete thinking and impaired abstraction |
| Executive function deficits¶ |
* Typically bizarre (i.e., unreal, other-worldly, or impossible). † Negative and cognitive symptoms of schizophrenia correlate with neuroimaging evidence of dorsolateral prefrontal cortex dysfunction. ‡ These are the 4 As of Bleuler. § Formal thought disorder (a disorder of the form of thought) is also considered a positive symptom. ¶ Executive functions are the ability to initiate, regulate, plan, and sequence activities. The negative symptoms (apathy, indecision) can represent impaired executive functions. |
Diagnosis
Accurate diagnosis of schizophrenia is often challenging because symptoms are nonspecific and progression to full illness is gradual. Relevant signs and symptoms must be present for at least 6 months before a diagnosis of schizophrenia can be made. Acute psychosis is a necessary but insufficient criterion for diagnosing schizophrenia. The diagnostic criteria for schizophrenia are symptomatic, functional, and time based, and they require exclusion of both medical and other psychiatric disorders that can mimic schizophrenia. Schizophrenia is largely a diagnosis of exclusion. The DSM-5 diagnostic criteria for schizophrenia require that two or more of the following be present for a significant portion of time during a 1-month period: delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, and negative symptoms (Box 1).2 At least one of the two symptoms must be delusions, hallucinations, or disorganized speech. The primary symptoms are rated based on their current severity (defined as most severe in the past 7 days) on a 5 point scale ranging from 0 (not present) to 4 (present and severe).
Schizophrenia also has subtypes defined exclusively by symptom predominance. Their validity remains controversial.
DSM-5 includes two prominent changes to the diagnostic criteria for schizophrenia: 1) elimination of the need to fulfill just one symptom in Criterion A if the patient has bizarre delusions and schneiderian first rank auditory hallucinations and 2) the adoption of a dimensional approach to rating severity.2
Differential Diagnosis
If symptoms are not specific and signs and symptoms do not last for 6 months as required for diagnosing schizophrenia, the clinician is obliged to eliminate other important diagnostic considerations. These include psychiatric disorders, substance use, and general medical disorders (Boxes 2 and 3).
Box 2. Psychiatric and Substance Use Disorders that Can Cause Acute Psychosis9
Psychiatric Bipolar disorder Major depression with psychotic features Schizophrenia Schizoaffective disorder Schizophreniform disorders Brief psychotic disorder Factitious disorder with psychological signs and symptoms Side effect of antidepressant medications Drug Abuse Drug Use
Drug Withdrawal
|
Adapted and modified from Hyman SE: Acute psychoses and catatonia. Hyman SE, Tesar GE (eds): Manual of Psychiatric Emergencies, 3rd ed. Boston, Little, Brown, 1994, p 145. |
Box 3. General Medical Conditions that Can Cause Acute Psychosis9
Industrial Exposures Acute intermittent porphyria Carbon disulfide Cushing’s syndrome Heavy metals Hypocalcemia and hypercalcemia Hypoglycemia Hypothyroidism and hyperthyroidism Neurologic Disorders Central nervous system neoplasm Early Alzheimer’s disease Encephalitis, meningitis, brain abscess Huntington’s disease Neurosyphilis Seizure disorder (temporal lobe epilepsy, postictal psychosis) Stroke (right thalamic, Wernicke’s aphasia) Wilson’s disease Nutritional Deficiencies Korsakoff’s psychosis (thiamine deficiency) Pellagra (niacin deficiency) Vitamin B12 deficiency Wernicke’s encephalopathy (thiamine deficiency) Systemic Illness with Central Nervous System Effects Hepatic encephalopathy HIV/AIDS encephalopathy Hypoxic encephalopathy Lupus cerebritis Pancreatic encephalopathy Paraneoplastic syndrome Toxic Reactions to Medications ACE inhibitors Anticholinergic agents Antihistamines Digitalis Dopaminergic agents (bromocriptine, levodopa, ropinirole, mirapex) Glucocorticoids Isoniazid NSAIDs (indomethacin, sulindac) Over-the-counter sleep aids Stimulants (methylphenidate [Concerta, Focalin, Metadate, Ritalin], dextroamphetamine [Dexedrine, Adderall])ACE, angiotensin-converting enzyme |
AIDS, autoimmune deficiency syndrome; HIV, human immunodeficiency virus; NSAID, nonsteroidal anti-inflammatory drug. Adapted and modified from Hyman SE: Acute psychoses and catatonia. Hyman SE, Tesar GE (eds): Manual of Psychiatric Emergencies, 3rd ed. Boston, Little, Brown, 1994, p 145. |
Acute psychosis, although not recognized as a diagnostic term in DSM-5 is commonly used to describe a rapid deterioration of behavior associated with hallucinations and delusions. Schizophreniform disorder, brief psychotic disorder, and organic psychoses fall under this rubric. The DSM-5 diagnosis of schizophreniform disorder depends on the persistence of schizophrenia-like symptoms for at least 1 month and exclusion of other causes of acute psychosis. Brief psychotic disorder (often referred to as brief reactive psychosis) lasts less than 1 month, but more than 1 day. It is typically regarded as a reaction to marked stress in persons with borderline or antisocial personality disorders.
Schizoaffective disorder is a chronic mental illness that includes prominent features of both schizophrenia and a mood disorder. The diagnostic criteria for schizoaffective disorder are characteristic symptoms of schizophrenia concurrent with a major mood disturbance (major depressive or manic episode). Although mood symptoms and episodes must be present for a substantial portion of the total course of the illness, a diagnosis of schizoaffective disorder also requires that psychotic symptoms, such as delusions or hallucinations, have been present for a minimum of 2 weeks in the absence of an active mood disturbance.
Delusional disorder is distinguished from schizophrenia by delusions (e.g., erotomanic, grandiose, jealous, persecutory, somatic, mixed) that are not bizarre and functioning that is not markedly impaired. Hallucinations are generally not present.
A diagnosis of mood disorder with psychotic features is made if psychotic symptoms occur solely during episodes of mood disturbance.
Acute psychosis caused by substance use or medication toxicity is distinguished from schizophrenia by clear-cut evidence of substance use leading to symptoms.
Treatment
Advertisement
General Approach
The successful treatment of schizophrenia requires simultaneous attention to medical variables and psychosocial factors relevant to the patient. A multimodal approach encompassing biologic and psychosocial therapies as well as programs that offer rehabilitation and social reintegration has been found to be most effective. Schizophrenia generally does not occur in isolation but rather with other comorbid conditions, commonly alcohol or drug abuse, or both. Failure to recognize and treat comorbid substance abuse is a common cause of treatment resistance in schizophrenia. Comprehensive management of schizophrenia, therefore, typically requires the involvement of a multidisciplinary team including a psychiatrist, social worker, case manager, individual or family therapist, and one or more family members. Episodes of illness can require treatment in multiple settings, including outpatient, intensive outpatient, hospital, and residential.
The primary care physician’s principal role is to recognize the illness, initiate treatment, and refer to a psychiatrist.
Treatment of schizophrenia is divided into three phases: acute, stabilization, and stable.3 Generally, the illness first comes to medical attention with the presentation of an acute psychotic episode. Acute psychosis, like schizophrenia, has a differential diagnosis that includes general medical, psychiatric, and substance-use disorders, (see Boxes 2 and 3). At this point the primary care physician’s role may be to ensure safe transfer of the acutely psychotic patient to an emergency facility where appropriate evaluation and stabilization can be conducted. Once the proper treatment regimen for a schizophrenic patient has been identified, the primary care physician may be called on to prescribe maintenance medication, with specialist referral for assistance in managing recurrent illness episodes.
Proper medical care is another important consideration in the comprehensive management of the schizophrenic patient. The patient’s idiosyncratic behavior, poor hygiene, or nonadherence to medical recommendations often interferes with attention to and successful management of medical problems. Schizophrenic patients have a high incidence of cardiovascular problems such as hypertension and coronary artery disease, diabetes, and tobacco-related disorders. Given that many schizophrenic patients are homeless, higher rates of tuberculosis, HIV infection, and problems associated with poor foot care are also common in this population.
Acute Phase: Treatment of Acute Psychosis
The first priority in management of acute psychosis is the safety of patient and staff. This includes simultaneous attention to potentially life-endangering causes of acute psychosis or delirium (Box 4) and other psychiatric, substance-use (see Box 2), and general medical (see Box 3) causes. Identification of the underlying cause of the acute psychosis requires a thorough evaluation that includes the patient’s psychiatric and medical histories; collateral information from the family, workplace, school, and other sources; physical and mental status examinations; and laboratory investigation. Typically, much of this information is either unavailable or difficult to obtain, and the clinician is forced to rely on rapid observation, clinical intuition, and laboratory measures (Table 1). A commonly raised question is whether or not to obtain brain neuroimaging. Most experts recommend CT or MRI during a first psychotic episode. An electroencephalogram should be obtained if one suspects organic psychosis such as delirium (encephalopathy). If an underlying cause of psychosis is discovered, it should be corrected (e.g., hypoglycemia, cerebral vasculitis, seizures). In the absence of a definitive cause, the focus can shift to pharmacologic intervention. Voluntary or involuntary hospitalization is often necessary for the first episode of psychosis in schizophrenia.
Box 4. Life-Endangering Causes of Acute Psychosis or Delirium: WWHHHIMPS
Withdrawl from alcohol or barbiturates Wernicke’s encephalopathy Hypertensive crisis Hypoglycemia Hypoxemia (cerebral) Intracranial process (tumor, stroke) Meningitis or encephalitis Poisoning (overdose, heavy metal toxicity) Seizures (postictal state, temporal lobe epilepsy) |
Table 1. Laboratory Investigation in Acute Psychosis and Chronic Schizophrenia
Test |
Acute Psychosis* |
Chronic Schizophrenia† |
| Blood glucose | × |
‡ |
| Blood glucose, fasting | × |
|
| Brain MRI or CT | × |
‡ |
| BUN | × |
‡ |
| CBC | × |
‡ |
| Creatinine | × |
‡ |
| ECG | × |
‡ |
| EEG | ‡ |
|
| Electrolytes | × |
‡ |
| Hepatitis B and C | ‡ |
‡ |
| HIV | ‡ |
‡ |
| Lipid profile | × |
|
| Liver function | × |
‡ |
| Pregnancy (women with childbearing potential) | × |
‡ |
| Syphilis (RPR, VDRL) | × |
‡ |
| Toxicology (serum and urine) | × |
‡ |
| Vitamin B12 and folic acid blood levels | × |
|
BUN, blood urea nitrogen; CBC, complete blood count; CT, computed tomography; ECG, electrocardiogram; EEG, electroencephalogram; HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory. *For all first-episode psychoses or acute psychoses in which the patient’s history is unknown. †Patients who have an existing diagnosis of schizophrenia and require regular monitoring of medical status. ‡Order only if the clinical situation warrants it or if the test result is considered important for routine monitoring of physical status. | ||
The traditionally accepted regimen for rapid control of agitation associated with acute psychosis is oral or intramuscular (IM) lorazepam (Ativan) 1 to 2 mg, alone or in combination with haloperidol (Haldol) 2 to 5 mg. Rapidly acting alternatives include oral or IM olanzapine (Zyprexa) 5 to 10 mg or ziprasidone (Geodon) 20 mg IM or 60 to 80 mg oral. Oral dispersible forms of olanzapine (Zyprexa Zydis) 5 to 10 mg and risperidone (Risperdal M-Tab) 1 to 2 mg are useful when rapid absorption is desired and for noncompliant patients who seek medication. Simultaneous administration of IM olanzapine and lorazepam is not recommended because the combination has been associated with respiratory failure. An algorithm for treating acute psychosis is presented in Figure 1.
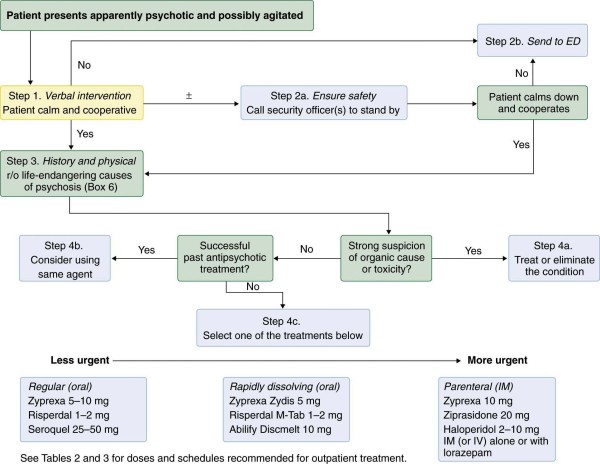
Figure 1 Algorithm for managing acute psychosis. ED, emergency department; r/o, rule out.
Pharmacologic Treatment of Schizophrenia
Antipsychotics are considered to be the first line of therapy in the pharmacologic treatment of schizophrenia. They are generally categorized as first-generation (typical) antipsychotics (FGAs) or second-generation (atypical) antipsychotics (SGAs). Typical starting and therapeutic doses of FGAs and SGAs are listed in Tables 2 and 3.
Table 2. First-Generation (Atypical) Antipsychotics
Drug |
Daily Dosage |
Preparations |
|||||||
Starting (mg/day) |
Range (mg/day) |
Maximum (mg/day) |
Schedule |
Pill, Capsule |
Elixir |
IM |
IV |
||
Short Acting |
Long Acting |
||||||||
| Chlorpromazine | 50-100 | 400-800 | 1000 | bid | × |
||||
| Thioridazine | 50-100 | 150-300 | 800 | bid-qid | × |
||||
| Trifluoperazine | 2-4 | 5-10 | 40 | bid | × |
||||
| Fluphenazine | 0.5-1 | 5-10 | bid | × |
|||||
| Fluphenazine decanoate | 12.5 | 25-50 | 100 | q 3-6 wk | × |
||||
| Perphenazine | 12-24 | 32-64 | 64 | tid-qid | × |
||||
| Molindone (Moban) | 50-75 | 50-200 | 225 | tid-qid | × |
× |
|||
| Loxapine | 10-20 | 60-100 | 250 | bid | × |
||||
| Thiothixene (Navane) | 6-10 | 5-15 | 60 | bid-tid | × |
||||
| Haloperidol | 2-10 | 5-15 | 100 | bid-tid | × |
||||
| Haloperidol lactate | 1-5 | 5-10 | 100 | bid-tid | × |
×a |
|||
| Haloperidol decanoate (Haldol Decanoate) | 25 | 50-100 | 450 | q mo | × |
||||
Note: First-generation antipsychotics are no longer considered first-line treatment for schizophrenia unless an atypical antipsychotic is not available, and then either haloperidol or chlorpromazine should be considered. a - Not approved by U.S. Food and Drug Administration for IV use, but off-label use is common when IV access is available. |
|||||||||
Table 3. Second-Generation (Atypical) Antipsychotics
Drug |
Daily Dosage |
Preparations |
|||||||
Starting (mg/day) |
Range (mg/day) |
Maximum (mg/day) |
Schedule |
Pill, Capsule |
Rapidly Dissolving |
Elixir |
IM |
||
Short Acting |
Long Acting |
||||||||
| Clozapine (Clozaril)a | 25 | 300-600 | 900 | bid | × |
||||
| Risperidone (Risperdal) | 1-2 | 4-8 | 16 | qd-bid | × |
× |
|||
| Risperidone (Risperdal M-Tab) | 1-2 | 4-8 | 16 | qd-bid | × |
||||
| Risperidone (Risperdal Consta) | 12.5b | 25b | 50b | q 2 wk | × |
||||
| Paliperidone (Invega) | 3-6 | 6-12 | 12 | qd | × |
||||
| Paliperidone palmitate (Invega Sustenna) | 234c | 117c | qmo | × |
|||||
| Olanzapine (Zyprexa) | 5-10 | 15-20 | 20 | qd-bid | × |
× |
|||
| Olanzapine (Zyprexa Zydis) | 5-10 | 15-20 | 20 | qd-bid | × |
||||
| Ziprasidone (Geodon) | 40-80 | 80-160 | 160 | bid | × |
× |
|||
| Quetiapine (Seroquel) | 50-100 | 400-800 | 800 | bid | × |
||||
| Aripiprazole (Abilify) | 5-10 | 15-30 | 40 | qd | × |
||||
| Lurasidone (Latuda) | 40 | 40-160 | 160 | qd | × |
||||
| Asenapine maleate (Saphris) | 10 | 20 | 20 | bid | × |
||||
| Iloperidone (Fanapt) | 2mg | 12-24 | 24 | bid | × |
||||
Note: All second-generation (atypical) antipsychotics have U.S. Food and Drug Administration approval for use in schizophrenia. a - Not considered a first-line agent. Requires weekly monitoring of white blood cell count (WBC) for the first 6 months of treatment and then biweekly thereafter for the duration of use. b - Initial dose , administered at recommended intervals. c - Initial dose with 156 mg administered 1 week later; maintenance doses (117 mg) administered monthly. |
|||||||||
A guideline for the pharmacologic management of schizophrenia is presented in Figure 2. The choice of antipsychotic drug, dosage, and desired route of administration is based on phase of treatment, intensity of agitation, adherence to treatment recommendations, history of response to antipsychotic medications, and antipsychotic side-effect profile.
The FGAs are broadly classified into the phenothiazines (e.g., chlorpromazine) and butyrophenones (e.g., haloperidol). The phenothiazines are more anticholinergic, cause more weight gain, and are more likely than butyrophenones to cause postural hypotension. Overdose is more likely to be fatal with phenothiazines than with butyrophenones. Haloperidol, the most widely prescribed butyrophenone, is associated with a high risk of all types of extrapyramidal symptoms (EPS). Although effective, the FGAs have fallen out of favor because of their side-effect profiles, especially their propensity to cause EPS (Box 5).
Box 5. Treatment of Extrapyramidal Symptoms
Acute Dystonia Benzotropine mesylate (Cogentin) 2 mg PO/IM/IV bid Diphenhydramine (Benadryl) 25-50 mg IM/IV Trihexyphenidyl (Artane) 2-5 mg PO bid-tid Akathisia Clonazepam 0.5 mg PO bid Lorazepam 1 mg PO tid Pramipexole 0.125-0.5 mg PO qd Propranolol 10-30 mg tid Ropinirole 1-4 mg PO qd Neuroleptic Malignant Syndrome Anticholinergics (benztropine, trihexyphenidyl) Dantrolene sodium Dopamine agonists (amantadine, bromocriptine, ropinirole, pramipexole) Parkinsonism Amantadine 100 mg PO bid Anticholinergics (benztropine, trihexyphenidyl) Pramipexole 0.125-0.5 mg PO qd Ropinirole 1-4 mg PO qd Tardive Dyskinesia No uniformly effective treatment Tardive Dystonia Anticholinergics are sometimes helpful Withdrawal Dyskinesia Resume same antipsychotic agent Neuroleptic-induced catatonia Anticholinergics (benztropine, trihexyphenidyl) Bromocriptine 10 mg PO tid Dopamine agonists (amantadine, bromocriptine, ropinirole, pramipexole) |
Advertisement
The SGAs affect several receptor types—serotonin, histamine, noraderenergic, and muscarinic—in addition to the D2 receptors.4 The multiplicity of receptors targeted by SGAs contributes to their efficacy and side-effect profiles. The results of the oft-cited Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated that FGAs and SGAs have similar efficacy, but both groups have potentially troublesome side effects that warrant careful monitoring and can disrupt otherwise effective treatment.4-6 Although SGAs are less likely to cause EPS, they are not risk free. Quetiapine is the least likely to cause EPS. The risk of other adverse effects varies among individual SGAs. Their relative risk profiles for different side effects are presented in Table 4. Clozapine (Clozaril), the first SGA to be developed and marketed, has retained its reputation for being the most effective of all antipsychotics at treating negative symptoms. Unfortunately, its tendency to cause bone marrow suppression, weight gain, and the metabolic syndrome also distinguishes it from the other SGAs. It is therefore used only selectively.
The use of antipsychotics in elderly patients with dementia-related psychosis has been associated with a 1.7-fold increase in risk of death. Most deaths are related to cardiovascular, cerebrovascular, or infectious (i.e., pneumonia) causes. This was initially thought to be an atypical antipsychotic–specific phenomenon, but studies have shown that FGAs may pose a similar risk.
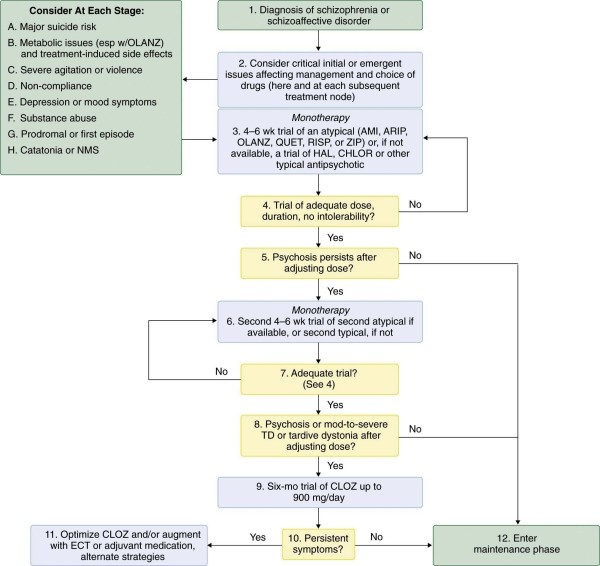
Figure 2 International Psychopharmacology Algorithm Project algorithm for schizophrenia. For descriptive not prescriptive purposes. AMI, amisulpride; ARIP, aripiprazole; CHLOR, chlorpromazine; CLOZ, clozapine; ECT, electroconvulsive therapy; esp, especially; HAL, haloperidol; NMS, neuroleptic malignant syndrome; OLANZ, olanzapine; QUET, quetiapine; RISP, risperidone; TD, tardive dyskinesia; ZIP, ziprasidone. Reprinted with permission from The International Psychopharmacology Algorithm Project. Copyright © 2004-2006 International Psychopharmacology Algorithm Project (IPAP) www.ipap.org.
Stabilization Phase
The dosage of medication used to achieve remission or optimal control in the acute phase should be continued for at least 6 months to prevent relapse. Psychotherapeutic interventions remain supportive. Patients should be helped with the transition to life in the community and helped to adjust to their lives outside the hospital through realistic goal setting.
Stable Phase
The goals of the stable phase are sustained symptom control or remission. Monthly to semiannual monitoring for treatment adherence, relapse, and intolerance to medications is recommended. Signs and symptoms of weight gain, increasing waist circumference, hyperlipidemia, and hyperglycemia should be monitored, as well as evidence of abnormal involuntary movements.7 The Abnormal Involuntary Movement Scale (AIMS) (Table 4) should be used serially to rate presence and intensity of movement disorder.
Table 4. Abnormal Involuntary Movement Scale8
Assessment
Rating Rate each item on a 0 to 4 scale for the greatest severity observed. Movements that occur only on activation merit 1 point less than those that occur spontaneously. |
|||||
Score |
|||||
Movement |
None |
Minimal* |
Mild |
Moderate |
Severe |
| Face and Mouth | |||||
| Muscles of facial expression | 0 | 1 | 2 | 3 | 4 |
| Lips and perioral area | 0 | 1 | 2 | 3 | 4 |
| Jaw | 0 | 1 | 2 | 3 | 4 |
| Tongue | 0 | 1 | 2 | 3 | 4 |
| Extremities | |||||
| Arms | 0 | 1 | 2 | 3 | 4 |
| Legs | 0 | 1 | 2 | 3 | 4 |
| Trunk | |||||
| Neck | 0 | 1 | 2 | 3 | 4 |
| Shoulders | 0 | 1 | 2 | 3 | 4 |
| Hips | 0 | 1 | 2 | 3 | 4 |
| Global | |||||
| Severity of abnormal movements | 0 | 1 | 2 | 3 | 4 |
| Incapacitation due to abnormal movements | 0 | 1 | 2 | 3 | 4 |
| Patient’s awareness of abnormal movements (0 = unaware; 4 = severe distress) | 0 | 1 | 2 | 3 | 4 |
| *May be the extreme of normal. | |||||
Continued antipsychotic treatment reduces the risk of symptom relapse. There are no strict guidelines for the minimum antipsychotic dose required to prevent relapse. For FGAs, the optimal dose is regarded as the minimum dose at which mild EPS are detectable on physical examination. SGAs can be administered at therapeutic doses well below their EPS threshold.
Pharmacologic treatment of schizophrenia is essential but insufficient. Optimal outcome requires additional use of psychosocial therapies and programs that foster recovery through vocational rehabilitation and social reintegration.
Psychosocial Interventions
Advertisement
Assertive Community Treatment
Developed in the late 1960s, assertive community treatment provides the patient with around-the-clock support in the community, thereby significantly reducing the time spent in hospitals. A team composed of a social worker, nurse, and case manager provides treatment in community settings. Services delivered include case management, initial and ongoing assessments, access to psychiatric services, employment and housing assistance, family support and education, substance-abuse services, and any other services and support critical to successful adaptation in the community.
Psychotherapy
The quality of the therapeutic alliance may be the best predictor of compliance and outcome. The emphasis is on education, support, and problem solving, rather than on developing insight. Therapy of this type can be provided on an individual or group basis.
Family Therapy
The schizophrenic patient’s behavior can trigger a vicious cycle of conflict between the patient and family. Anger, criticism, and devaluing comments directed by family members at the patient—referred to in the literature as high expressed emotion—are associated with a greater increase of relapse even when pharmacologic management is optimal. A therapist works with the family to reduce expressed emotion by educating them about schizophrenia and helping to modify the behaviors and attitudes that undermine the patient.
Social Skills Training
The principles of learning theory are used to improve social skills such as interpersonal relationships, employment, and leisure. Behaviors such as odd facial expressions, lack of spontaneity, and inappropriate perception of others’ emotional states are targeted and modified.
Vocational Rehabilitation
Workshops and part-time employment programs help the patient acquire greater functionality.
Screening and Prevention
Most prevention efforts are in the realm of secondary and tertiary prevention, or reducing the number and severity of episodes. Public health education on schizophrenia helps to reduce stigma and resistance to seeking treatment. Family history of schizophrenia is an important indicator of risk that should increase vigilance for early detection and treatment of prodromal symptoms. Once the diagnosis is made, the team should develop a comprehensive treatment plan that includes family involvement with goals of adhering to treatment and reducing symptoms. Assertive community treatment has been very effective at maintaining community and keeping patients out of the hospital. Assiduous attention to substance abuse and abstinence is a key to a good outcome in schizophrenia.
Considerations in Spceial Populations
Populations with special needs include patients with pervasive developmental disorders and mental retardation, women with childbearing potential, children, the elderly, and the homeless.
Summary
- Schizophrenia is a treatable neuropsychiatric disorder present in approximately 1% of the general population.
- The etiology is multifactorial and includes genetic, developmental, and possibly environmental causes.
- The signs and symptoms of schizophrenia are nonspecific, warranting a thorough evaluation for other medical and psychiatric disorders that can manifest with psychosis.
- The primary care physician should be familiar with the use, benefits, and potential adverse effects of antipsychotic medications used to treat schizophrenia.
- Metabolic syndrome is a common comorbidity, especially since the introduction of atypical (second-generation) antipsychotics.
References
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Lehman AF, Lieberman JA, Dixon JA, et al. Practice guideline for the treatment of patients with schizophrenia. 2nd ed. Psychiatryonline website. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1665359. Published February 2004. Accessed August 2, 2013.
- Markowitz JS, Brown CS, Moore TR. Atypical antipsychotics. Part I: pharmacology, pharmacokinetics and efficacy. Ann Pharmacother 1999; 33:73–85.
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19(suppl 1):1–93.
- Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223.
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27:596–601.
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health, Education and Welfare; 1976.
- Hyman SE. Acute psychoses and catatonia. In: Hyman SE, Tesar GE, eds. Manual of Psychiatric Emergencies. 3rd ed. Boston, MA: Little, Brown; 1994:143–157.
Suggested Readings
- Kaplan HI, Sadock BJ, eds. Comprehensive Textbook of Psychiatry. 6th ed. Vol 1. Baltimore, MD: Williams & Wilkins; 1995:984–987.
- Rossler W, Salize HJ, van Os J, Riecher-Rossler A. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol 2005; 15:399–409.
